Hassle-Free Monitoring of Global Standards, Regulations and Guidelines

In regulated industries, Regulations, Standards, and Guidelines build the basis for products and compliance.
-
But what if these change?
-
Who knows that they have changed?
We have the solution and all the answers centralized in one single platform.
What is it?
PREXEVUS is an advanced standards, guideline and regulations monitoring system using proprietary artificial intelligence and analytics tools to bring a 360° compliance control to the user. PREXEVUS supports state-of-the-art claims by alerting in cases of updates or withdrawn standards, guidelines or regulations tailored to the products and services in the client’s markets.
Why using it?
PREXEVUS goes the extra mile in providing not only monitoring and alerting, but bringing you the essence of the changes and adoptions in the regulatory field. Paired with on-demand training and consultancy, PREXEVUS offers a real 360° support for Regulatory Experts and the Regulatory Intelligence for Development, Product Maintenance and Market expansion.
What’s different?
PREXEVUS offers an advanced monitoring of standards, regulations and guidelines with a global focus. Proprietary data mining and Natural Language Processing (NLP)-algorithms allow for fast and accurate interpretation of new or changed texts.
PREXEVUS is the Robo-Advisor in Regulatory Intelligence.
The Product
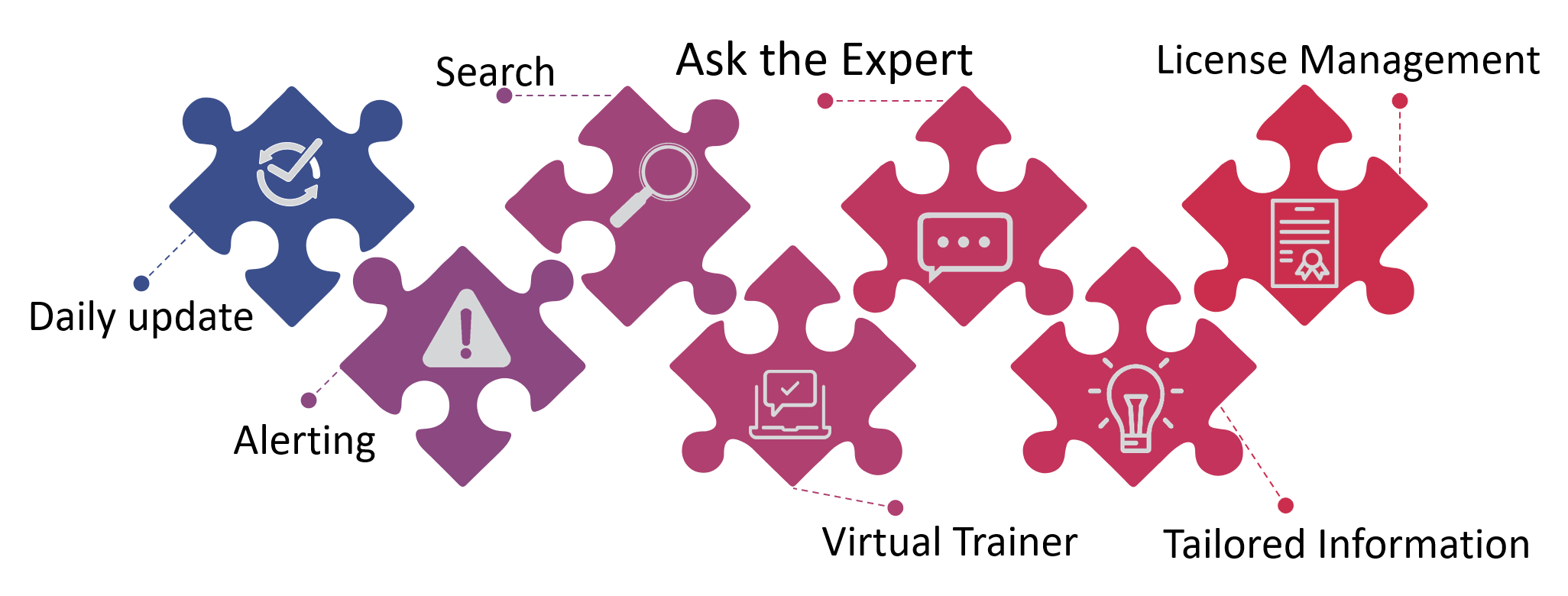
The platform of PREXEVUS combines advanced compliance monitoring, specific alerting, document management, contextual analysis, learning and support.
All in a modular way, pre-set and ready to use.
PREXEVUS can be used for:
- Medical Devices
- Pharmaceuticals
- Cosmetics
- Novel Food
- and any other regulated industry.
The Features

Monitoring – The monitoring is done based on your listed standards, guidelines and regulations in real time and currently for ISO, IEC, EN, ASTM standards as well as guidelines and regulations from EC and US-FDA… and we keep adding more and more

Standards – Via PREXEVUS, you can buy the standards you really need and there is no need to buy packages with irrelevant standards to your business.

Standards Management – Stop spending money on duplicate standards. We manage your relevant standards in a simple but cost-effective way.

Virtual Trainer – Stop struggling with the complex content of a standard, guideline or regulation and take the virtual training course from PREXEVUS.

Alerting – In the case of changes to your selected standards, guidelines and regulations, PREXEVUS alerts instantly keeping the information tailored and relevant to your business needs.
Expert Help – Monitoring and virtual training build the basis for a general understanding but your business needs a tailored service for support? Ask for an expert relevant to the standard, guideline or regulation.
Sign-up now!
PREXEVUS is your Robo-Advisor in Regulatory Intelligence.
Put your compliance to automation now.
The Monitoring

ISO – We monitor all Standards from the International Standards Organization. ISO has currently published > 22’000 Standards, constantly evolving to meet and standarize the technological advances.

IEC – We monitor all Standards from the International Electrotechnical Commission. The IEC has currently published > 11’000 documents, constantly evolving to meet and standardize the technological advances.

ASTM – We monitor all Standards from the American Society for Testing and Materials. ASTM has currently published > 12’000 Standards, constantly evolving to meet and standardize the technological advances.

MEDDEV – We monitor all Guidelines from the Medical Device (MEDDEV) repository.

FDA – We monitor all Guidelines and consensus Standards from the United States FDA

EN – We monitor all Standards from the European Standards. With this, we can highlight whether an ISO standard is harmonized by EN for Europe.

CENELEC – We monitor all Standards from the European Committee for Electrotechnical Standardization. With this, we can highlight whether an IEC standard is harmonized by CEN for Europe.

FDA – We monitor all United States FDA Regulations from 21 Code of Federal Regulations.

EC – We monitor all European Commission Regulations.
